american academy of pediatrics covid vaccine under 12
We recently sat down with Nina Alfieri MD MS FAAP Advanced General Pediatrics and Primary Care to answer commonly asked questions and to address concerns from parents and caregivers about the COVID-19 vaccine for children under 12. Limiting screen time also helps parents keep a closer eye on what their children are experiencing on social media and the internet.
/cdn.vox-cdn.com/uploads/chorus_asset/file/22273790/harlem_vaccine_site.jpg)
Will New York Require Covid Vaccines For Students State Pediatricians Hope So The City
If not vaccination or re-vaccination is standard of care checking serologic titers is an alternative as the risks from non-vaccination are greater than re-vaccination.
. Vaccine trial data on efficacy safety. The Biden administration released the Path Out of the Pandemic COVID-19. The COVID shot for kids 5 years to 11 years of age is a lower dose than the dose recommended for people 12 years and older.
AAP updates treatment guidance Health officials also shortened the time interval for boosters after a Pfizer-BioNTech primary series and will allow a third primary series dose for certain immunocompromised children ages 5-11 years. Pfizer is a mRNA vaccine and requires two doses injected three weeks apart. Children 511 will get a 10-microgram dose compared to the 30-microgram dose that adults and children 12 and older receive.
Visit the Growth Chart Training website for a set of self-directed interactive training courses. Children under 20 years of age. The American Academy of Pediatrics Centers for Disease Control and Prevention and many academic pediatricians advise against this Muller says.
In Ohio nearly 206000 children have contracted COVID-19 and 15. Despite decreasing cases of COVID-19 some children and many adults are still getting extremely sick and. Is the Pfizer BioNTech mRNA vaccine.
Grummer-Strawn LM Reinold C Krebs NF. FDA authorizes COVID boosters for youths ages 12-15. Many parents that jumped at chance to get themselves.
Vaccinating children against coronavirus disease 2019 COVID-19 is critical as a public health strategy to reach herd immunity and prevent illness among children and adults. If approved the pediatric vaccine would be given in two 10-microgram doses administered 21 days apart. HealthDayIn guidance issued by the American Academy of Pediatrics recommendations are presented to help pediatricians support the emotional and behavioral needs of children teenagers and.
Studies show that a high level of immunity is reached about two weeks after receiving the vaccines second dose. 1 In the first stages of the COVID-19 pandemic mid-2020 before any vaccine was available and when parents of children of all ages 018 years old completed a survey close. Nearly 1 million kids under age 12 have had the Covid vaccine WH says.
The American Academy of Pediatrics AAP reported that since the beginning of the pandemic about 19 million children ages 5-11 years have been infected with COVID-19 which is about 9 of all US. For children older than 2 years 2 to 19 years of age CDC and the American Academy of Pediatrics recommend that health care providers use the CDC Growth Reference Charts. Last week nearly 170000 children tested positive for COVID-19 up by about 28 in the last two weeks according to a report released Monday by the American Academy of Pediatrics and the Children.
28 2021 nearly 64 million Americans younger than 18 years had been infected with COVID-19 and 791 had died. The dosage is one-third of the adolescent and adult dose. Age vaccine dose.
From car seats and vaccines to screen time and obesity the American Academy of Pediatrics AAP routinely publishes guidelines and advice to help parents keep their kids safe and healthy. The Pfizer COVID vaccine for 5 to11 year olds has been approved and recommended by the FDA CDC and American Academy of Pediatrics. With COVID-19 cases decreasing in the US will children under 12 need to get a vaccine.
Right now the only COVID-19 vaccine available for children in the US. Of children infected more than 8300 children have been hospitalized and 94 have died according to federal data. A single dose of vaccine given at 12 to 15 months of age has an efficacy of 95.
The American Academy of Pediatrics answers questions parents may have about the COVID-19 vaccine. Clinical trials in children ages 5-11 years found the vaccine to be 907 effective in preventing symptomatic COVID-19. Even if your 45-year-old is the size of a 5-year-old their immune system can go through a lot of important development in those next six months he says.
In fact there is likely an AAP policy statement for. Haga clic aquí para ver esta información en español. One hour per day for children 2 to 12.
Sean OLeary vice chair of the committee on infectious diseases for the American Academy of Pediatrics said he. Vaccination records from foreign countries can be accepted if doses are appropriately timed and credible-appearing documentation is present. According to the American Academy of Pediatrics as of Oct.
Learn how it safely teaches your childs immune system to recognize and get rid of the coronavirus that causes COVID-19. Fryhofer chair-elect the AMA. Recent federal vaccine mandate regulations and ongoing litigation are key to the vaccine mandate landscape and a significant influence on state policy.
The American Academy of Pediatrics is on board with vaccinating this age group along with the American Academy of Family Physicians and the Pediatrics Infectious Diseases Society said Dr. Beacon Pediatrics is pleased to announce that another milestone has occurred in the fight against COVID-19. On Modernas COVID-19 vaccine for 12- to 17-year-olds.
If the skin test is negative the vaccine can be readministered under close observation with resuscitation including epinephrine readily available10 12 Every effort should be made to avoid. Two hours per day for teens and adults. Since the start of the pandemic representing 172 of cases overall.
At this time the Pfizer vaccine is the only COVID-19 vaccine authorized for those age 5 and older. Walgreens and CVS have started administering COVID vaccines to children under 12 years old. No screen time for children under 2.
The American Academy of Pediatrics reported that more than 7 million children have had COVID in the US. The American Academy of Pediatrics recommendations for an acceptable amount of screen time are. COVID-19 vaccination is recommended for children 5 years and older.
The vaccine has been found to be very effective in preventing COVID disease in 5 to 11 year old children as well as. The American Academy of Pediatrics says.
The Pfizer Covid 19 Vaccine For Kids Is Coming Here Are 6 Things To Know

What Parents Need To Know About Covid 19 Vaccines For Children Johns Hopkins Bloomberg School Of Public Health
Kids Covid Vaccine Side Effects What To Know

Covid Vaccines For Kids Under 12 A Breakdown Of What You Need To Know Nbc Chicago

When Will A Covid 19 Vaccine Be Ready For Kids Under 12 And What S The Latest News On Clinical Trials Connecticut Children S

Kids Covid Vaccine Side Effects What To Know
Vaccinating Children Ages 5 11 Policy Considerations For Covid 19 Vaccine Rollout Kff
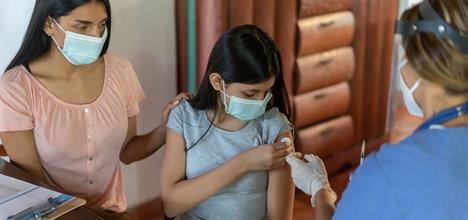
Aap Advises Children And Teens Age 12 And Up Get The Covid 19 Vaccine Healthychildren Org

Pfizer Gets Fda Approval To Enroll Kids As Young As 12 In Covid 19 Vaccine Trial Shots Health News Npr

The Covid 19 Vaccination What Parents Of Children 5 11 Need To Know

Pediatricians Want Kids To Be Part Of Covid Vaccine Trials Kaiser Health News
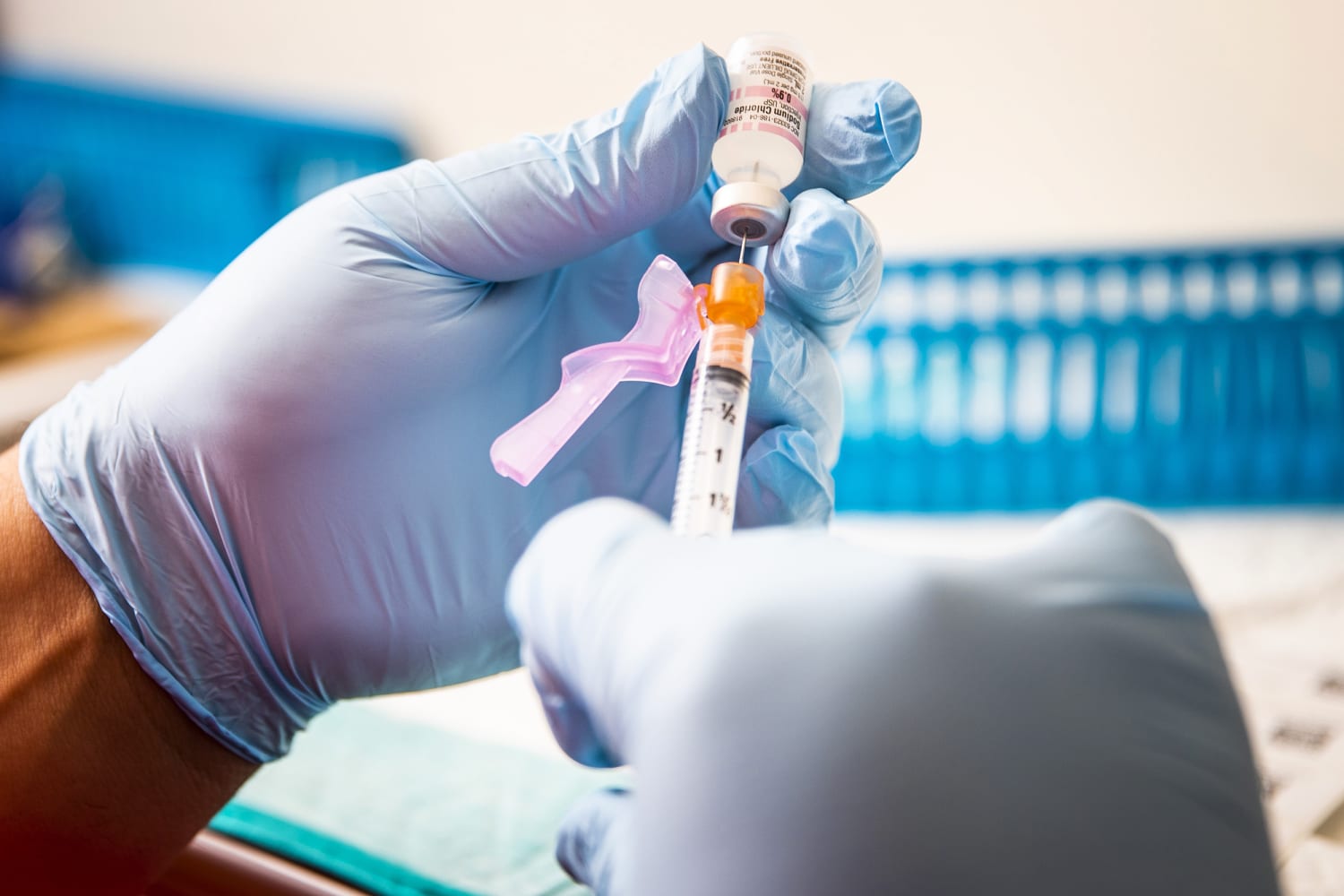
Covid Vaccines For Kids Under 12 Expected Midwinter Fda Official Says

Should My Child Get The Covid 19 Vaccine Baton Rouge Clinic

Fda Again Warns Parents Not To Get Children Under 12 Vaccinated Yet The New York Times

More Children Are Catching Covid 19 But It S Unclear If They Re Getting Sicker Coronavirus Updates Npr

What Parents Should Know About Covid 19 Vaccines For Kids Under 12

Covid Vaccines In Teens And Heart Inflammation What You Need To Know Shots Health News Npr
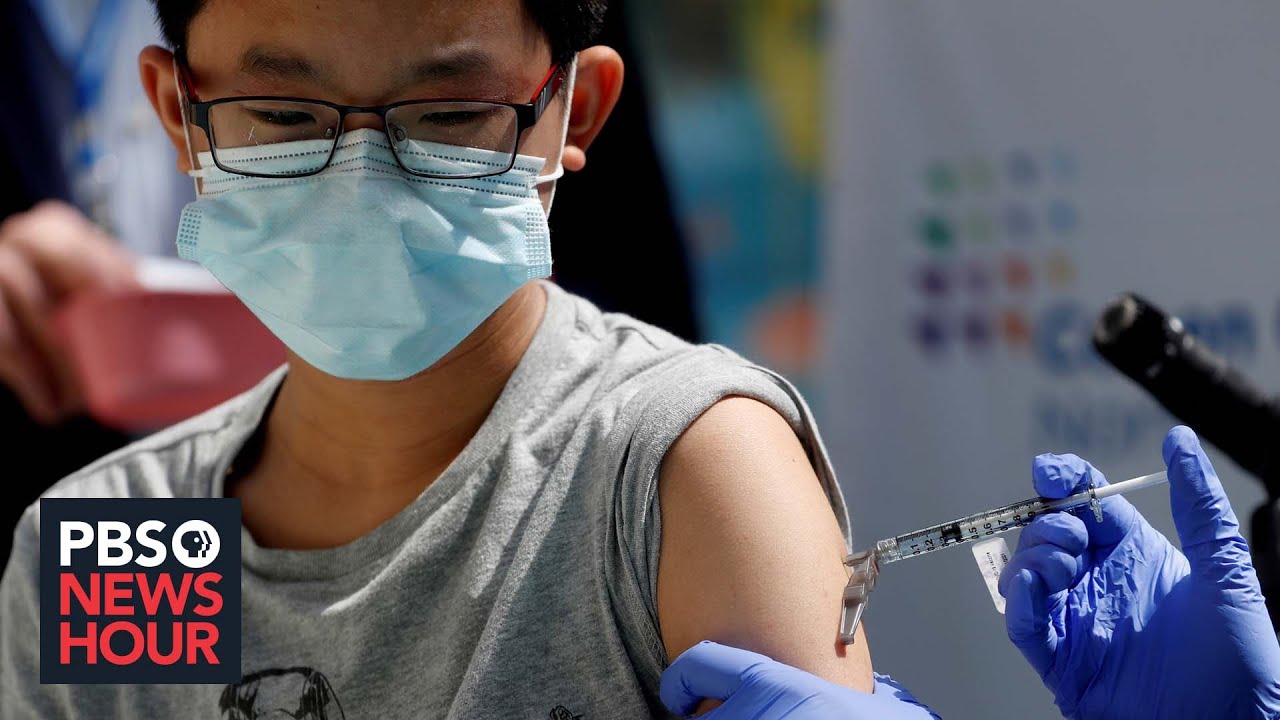
American Academy Of Pediatrics Urges Fda To Approve Covid Vaccines For Children Under 12 Youtube

Cdc Is Investigating Heart Problems In A Few Young Covid 19 Vaccine Recipients The New York Times